Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Sr in highly radioactive aqueous samples via conversion to a kinetically stable 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid complex followed by concentration-separation-fractionation based on capillary electrophoresis-liquid scintillation
Sr in highly radioactive aqueous samples via conversion to a kinetically stable 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid complex followed by concentration-separation-fractionation based on capillary electrophoresis-liquid scintillationOuchi, Kazuki; Haraga, Tomoko; Hirose, Kazuki*; Kurosawa, Yuika*; Sato, Yoshiyuki; Shibukawa, Masami*; Saito, Shingo*
Analytica Chimica Acta, 1298, p.342399_1 - 342399_7, 2024/04
Given that conventional methods of high-dose sample analysis pose substantial exposure risks and generate large amounts of secondary radioactive waste, faster procedures allowing for decreased radiation emission are highly desirable. To address this need, we developed a  Sr
Sr quantitation technique that is based on liquid scintillation counting-coupled capillary transient isotachophoresis (ctITP) with two-point detection and relies on the rapid concentration, separation, and fractionation of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-complexed
quantitation technique that is based on liquid scintillation counting-coupled capillary transient isotachophoresis (ctITP) with two-point detection and relies on the rapid concentration, separation, and fractionation of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-complexed  Sr
Sr in a single run. This method, which allows for the handling of high-dose radioactive specimens at the microliter level and is substantially faster than conventional ion-exchange protocols, was used to selectively quantify
in a single run. This method, which allows for the handling of high-dose radioactive specimens at the microliter level and is substantially faster than conventional ion-exchange protocols, was used to selectively quantify  Sr
Sr in real high-dose waste. The successful concentration-separation in ctITP was ascribed to the inertness of the Sr-DOTA complex to dissociation.
in real high-dose waste. The successful concentration-separation in ctITP was ascribed to the inertness of the Sr-DOTA complex to dissociation.
 -edge for Ce in bauxites using transition-edge sensors; Implications for Ti-rich geological samples
-edge for Ce in bauxites using transition-edge sensors; Implications for Ti-rich geological samplesLi, W.*; Yamada, Shinya*; Hashimoto, Tadashi; Okumura, Takuma*; Hayakawa, Ryota*; Nitta, Kiyofumi*; Sekizawa, Oki*; Suga, Hiroki*; Uruga, Tomoya*; Ichinohe, Yuto*; et al.
Analytica Chimica Acta, 1240, p.340755_1 - 340755_9, 2023/02
Times Cited Count:2 Percentile:31.9(Chemistry, Analytical)no abstracts in English
Haraga, Tomoko; Ouchi, Kazuki; Sato, Yoshiyuki; Hoshino, Hitoshi*; Tanana, Rei*; Fujihara, Takashi*; Kurokawa, Hideki*; Shibukawa, Masami*; Ishimori, Kenichiro; Kameo, Yutaka; et al.
Analytica Chimica Acta, 1032, p.188 - 196, 2018/11
Times Cited Count:12 Percentile:45.99(Chemistry, Analytical)The development of safe, rapid and highly sensitive analytical methods for radioactive samples, especially actinide (An) ions, represents an important challenge. Here we propose a methodology for selecting appropriate emissive probes for An ions with very low consumption and emission of radioactivity by capillary electrophoresis-laser-induced fluorescence detection (CE-LIF), using a small chemical library of probes with eight different chelating moieties. It was found that the emissive probe, which possesses the tetradentate chelating moiety, was suitable for detecting uranyl ions. The detection limit for the uranyl-probe complex using CE-LIF combined with dynamic ternary complexation and on-capillary concentration techniques was determined to be 0.7 ppt. This method was successfully applied to real radioactive liquid samples collected from nuclear facilities.
Okubo, Ayako; Obata, Hajime*; Magara, Masaaki; Kimura, Takaumi; Ogawa, Hiroshi*
Analytica Chimica Acta, 804, p.120 - 125, 2013/12
Times Cited Count:2 Percentile:7.47(Chemistry, Analytical)This work introduces a novel method of recovery of iron hydroxide using a DIAION CR-20 chelating resin column to determine Th isotopes in seawater with a sector field (SF) inductively coupled plasma mass spectrometer (ICP-MS). Thorium isotopes in seawater were coprecipitated with iron hydroxide, and this precipitate was sent to chelating resin column. The chelating column quantitatively collected 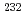 Th with iron hydroxide in seawater at flow rates of 20-25 mL/min. Based on this flow rate, a 5 L sample was processed within 3-4 h.
Th with iron hydroxide in seawater at flow rates of 20-25 mL/min. Based on this flow rate, a 5 L sample was processed within 3-4 h.
Esaka, Fumitaka; Lee, C. G.; Magara, Masaaki; Kimura, Takaumi
Analytica Chimica Acta, 721, p.122 - 128, 2012/04
Times Cited Count:27 Percentile:68.19(Chemistry, Analytical)A fission track technique was used as a sample preparation method for subsequent isotope abundance ratio analysis of individual uranium containing particles with secondary ion mass spectrometry (SIMS) to measure the particles with higher enriched uranium efficiently. This method was then applied to the analysis of a real inspection swipe sample taken at a nuclear facility. As a consequence, the range of  U/
U/ U isotope abundance ratio between 0.0276 and 0.0438 was obtained, which was higher than that measured by SIMS without using a fission track technique (0.0225 and 0.0341). This indicates that the fission track - SIMS method is a powerful tool to identify the particle with higher enriched uranium in environmental samples efficiently.
U isotope abundance ratio between 0.0276 and 0.0438 was obtained, which was higher than that measured by SIMS without using a fission track technique (0.0225 and 0.0341). This indicates that the fission track - SIMS method is a powerful tool to identify the particle with higher enriched uranium in environmental samples efficiently.
 -tetraalkylderivatives of diglycolamide-monoamide/n-dodecane media
-tetraalkylderivatives of diglycolamide-monoamide/n-dodecane mediaSasaki, Yuji; Sugo, Yumi; Suzuki, Shinichi; Kimura, Takaumi
Analytica Chimica Acta, 543(1-2), p.31 - 37, 2005/07
Times Cited Count:106 Percentile:93.99(Chemistry, Analytical)The detailed study of extraction capacity of diglycolamide (DGA) with and without DHOA, N,N'-dihexyloctanamide as a modifier, was performed by using the novel analytical method for the determination of the limit of metal concentration in the organic phase (LOC). The effect on modifier was studied and it is confirmed that LOC increases with DHOA concentration. It is noteworthy that LOC reaches to the stoichiometric value, which is obtained by the extraction reaction, by using 0.1M TODGA mixed with more than 0.5M DHOA into n-dodecane. Without modifier, relation of the different DGA compounds with LOC was investigated, and it is clear that LOC using N,N,N',N'-tetradodecyl-diglycolamide (TDdDGA), whose extractant can suppress the third phase, reaches to the stoichiometric value. From this work, it is obvious that the application of the condition without the third phase formation, e.g., enough concentration of modifier coexisted and DGA with long alkyl chain, is important to attain the stochiometric LOC value.
Aoyagi, Hisao*; Kitatsuji, Yoshihiro; Yoshida, Zenko; Kihara, Sorin*
Analytica Chimica Acta, 538(1-2), p.283 - 289, 2005/05
Times Cited Count:15 Percentile:41.62(Chemistry, Analytical)Redox behavior of Np(III), (IV), (V) and (VI) ions in aqueous perchloric, nitric and sulphuric acid solutions was elucidated by using column electrodes connected in series in a flow system. Using a glassy carbon fiber working electrode as the column electrode was found to be very useful for the investigation of current-potential relations of not only reversible redox processes such as Np(VI)/(V) or Np(IV)/(III) but also irreversible processes such as Np(V)/(IV) or Np(V)/(III), the latter not observed by conventional voltammetry which uses a glassy carbon or platinum electrode. Quantitative electrolysis could be executed even if the redox process was completely irreversible by the use of the column electrodes. By taking advantage of column electrode electrolysis, a novel method was developed for the rapid preparation of a neptunium species of a desired oxidation state, including unstable species. The multi-step column electrode system was demonstrated to be useful for the coulometric determination and speciation of Np(IV), (V) and (VI) in aqueous HNO solution as an example.
solution as an example.
 -tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into
-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into  -dodecane
-dodecaneZhu, Z.-X.; Sasaki, Yuji; Suzuki, Hideya*; Suzuki, Shinichi; Kimura, Takaumi
Analytica Chimica Acta, 527(2), p.163 - 168, 2004/12
Times Cited Count:263 Percentile:99.08(Chemistry, Analytical)The extraction of 75 elements by  -tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid to
-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid to  -dodecane was investigated and discussed concerning distribution ratios (D) of metal ions and their ionic radii. The extractability for elements depends strongly on their oxidation state. Monovalent and pentavalent ions were not extractable. In divalent metal extraction, Ca(II) (ionic radius: 100 pm) showed the highest D value and the D decreased with increase or decrease of the ionic radius. Tri- and tetravalent ions with ionic radii of 87-113 pm and 83-94 pm, respectively, showed the D over 1000, and the extracted chemical forms were determined to be M(TODGA)
-dodecane was investigated and discussed concerning distribution ratios (D) of metal ions and their ionic radii. The extractability for elements depends strongly on their oxidation state. Monovalent and pentavalent ions were not extractable. In divalent metal extraction, Ca(II) (ionic radius: 100 pm) showed the highest D value and the D decreased with increase or decrease of the ionic radius. Tri- and tetravalent ions with ionic radii of 87-113 pm and 83-94 pm, respectively, showed the D over 1000, and the extracted chemical forms were determined to be M(TODGA) (NO
(NO )
)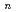 or M(TODGA)
or M(TODGA) (NO
(NO )
)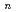 (
( =3 or 4). The oxonium ions with higher oxidation states than tetravalent one forms the complicated metal-complex with TODGA, and the number of TODGA associated in those extraction reactions was confirmed to be 2 at most.
=3 or 4). The oxonium ions with higher oxidation states than tetravalent one forms the complicated metal-complex with TODGA, and the number of TODGA associated in those extraction reactions was confirmed to be 2 at most.
Lee, C. G.; Iguchi, Kazunari; Esaka, Konomi; Magara, Masaaki; Esaka, Fumitaka; Sakurai, Satoshi; Watanabe, Kazuo; Usuda, Shigekazu
Analytica Chimica Acta, 517(1-2), p.215 - 220, 2004/07
Times Cited Count:4 Percentile:13.8(Chemistry, Analytical)In this study, we discussed the feasibility of solid sample ETV-ICP-MS for the particle analysis of the environmental swipe samples in order to establish the fission track-ETV-ICP-MS method. Samples used were solid samples containing Tl, Pb and U in polycarbonate film, and their standard solutions. The analytical performance will be reported in terms of the optimization of temperature program, and sensitivity and precision in isotope ratio measurement.
Takeishi, Hideyo; Kitatsuji, Yoshihiro; Kimura, Takaumi; Meguro, Yoshihiro; Yoshida, Zenko; Kihara, Sorin*
Analytica Chimica Acta, 431(1), p.69 - 80, 2001/03
Times Cited Count:31 Percentile:67.85(Chemistry, Analytical)no abstracts in English
Kitatsuji, Yoshihiro; Aoyagi, Hisao; Yoshida, Zenko; Kihara, Sorin*
Analytica Chimica Acta, 387, p.181 - 187, 1999/00
Times Cited Count:11 Percentile:39.77(Chemistry, Analytical)no abstracts in English
Hiyama, Toshiaki; Hiyama, Toshiaki; Takahashi, Toshio; Kamimura, Katsuichiro
Analytica Chimica Acta, 345(1), p.131 - 137, 1997/06
Times Cited Count:12 Percentile:44.06(Chemistry, Analytical)None
Kuno, Yusuke; Sato, Soichi; Masui, Jinichi
Analytica Chimica Acta, 270(1), p.181 - 186, 1992/12
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)None
Handa, Nuneo; Shiozawa, Kenichi; Iwai, Takashi; Arai, Yasuo
Analytica Chimica Acta, 239, p.107 - 113, 1990/00
Times Cited Count:9 Percentile:47.03(Chemistry, Analytical)no abstracts in English
Adachi, Takeo; Takeishi, Hideyo; Sasaki, Yuji; *
Analytica Chimica Acta, 218, p.77 - 84, 1989/00
Times Cited Count:18 Percentile:69.75(Chemistry, Analytical)no abstracts in English
*; *; ; ;
Analytica Chimica Acta, 183, p.217 - 223, 1986/00
Times Cited Count:20 Percentile:71.46(Chemistry, Analytical)no abstracts in English
Analytica Chimica Acta, 183, p.225 - 230, 1986/00
Times Cited Count:5 Percentile:33.11(Chemistry, Analytical)no abstracts in English
;
Analytica Chimica Acta, 172, p.39 - 47, 1985/00
Times Cited Count:7 Percentile:43.07(Chemistry, Analytical)no abstracts in English
Analytica Chimica Acta, 147, p.417 - 421, 1983/00
Times Cited Count:11 Percentile:64.37(Chemistry, Analytical)no abstracts in English
Fujino, Takeo; ;
Analytica Chimica Acta, 147, p.423 - 428, 1983/00
Times Cited Count:1 Percentile:11.06(Chemistry, Analytical)no abstracts in English